4: Growth mechanisms
Once stable crystal nuclei have formed, given the right conditions there is the opportunity for continued growth. Different growth mechanisms are dominant under different conditions. For solution-grown crystals, the main variable is the degree of supersaturation. Broadly speaking, there are three important regimes:
• Defect growth
• Two-dimensional growth
• Adhesive growth
At low supersaturation, defect growth is dominant. The sticking probability for a clean flat crystal surface is small, so deposition is unlikely. The presence of defects, however, possibly resulting from impurities or other irregularities, can dramatically increase the sticking probability by lowering the free energy for adhesion. In the diagram (below left), a step has formed as a result of a kink in the surface caused by a screw dislocation. The sticking probability at the step is dramatically higher because the energy barrier to sticking, ΔGs, is much lower. As the step advances forwards, it leaves the kink behind at the screw defect (below right).
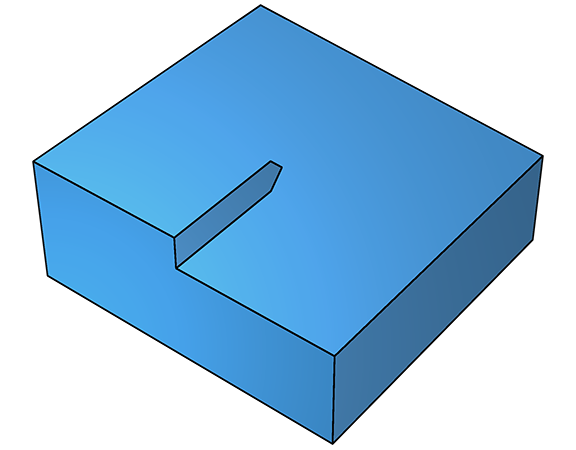
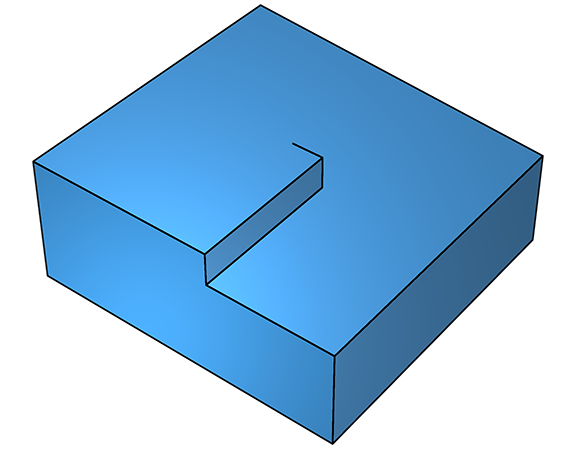
On further deposition, growth continues and a fresh step emerges, which grows along a new trajectory, but still parallel to the surface (below left). As this second step propagates, it too leaves the defect in its wake, leading to yet another step (below right).
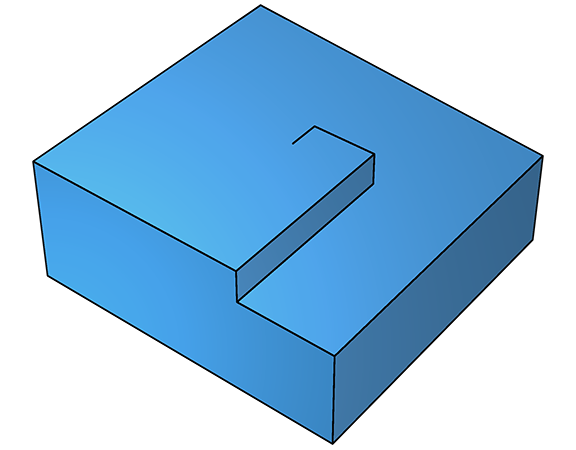
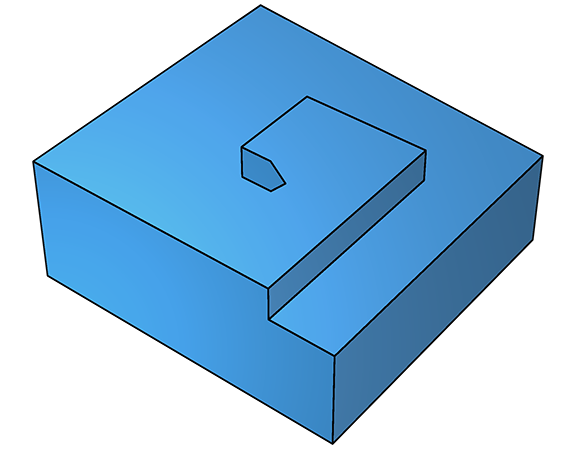
As this continues, the net effect is crystal growth that spirals around the screw defect. Spiral crystal growth is observable in real time using an atomic-force microscope.
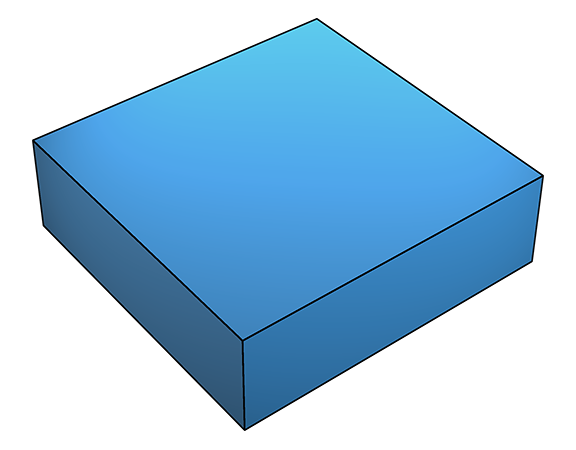
At higher supersaturation, deposition onto flat crystal surfaces (below left) occasionally becomes possible because the higher supersaturation β (see previous section) lowers ΔGs to the point where deposition can occur on an otherwise clean, flat surface (below right).
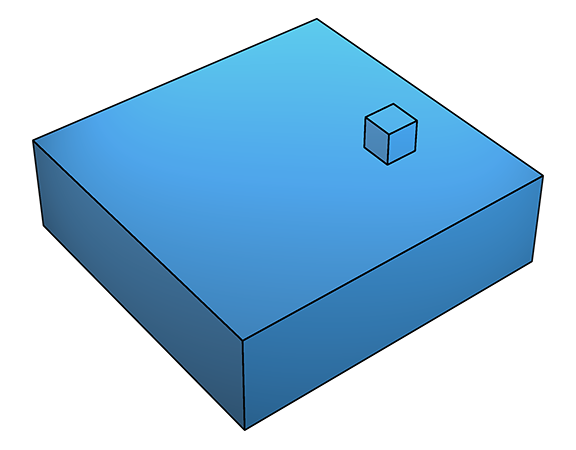
This leads to two-dimensional growth, also known as 'birth-and-spread' growth (below), as subsequent deposition tends to occur at the newly formed steps.
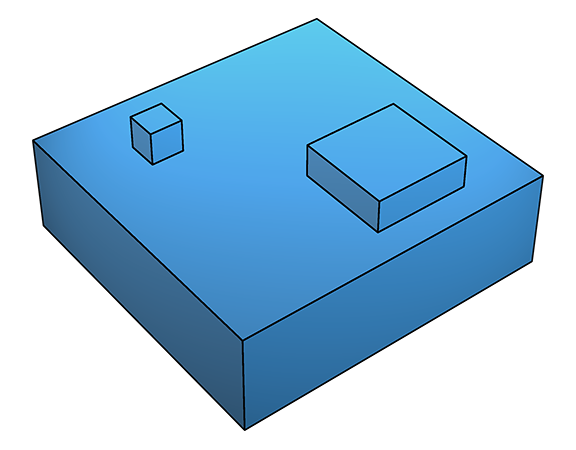
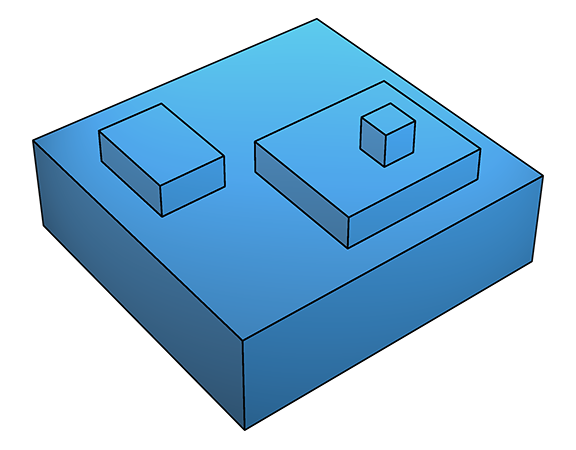
Two-dimensional growth is fastest for crystal surfaces that have high sticking probability, thus stepped surfaces (higher index) tend to grow faster than flatter (low index) surfaces.
Both the spiral-defect and two-dimensional growth mechanisms are characterized by incorporation of new material primarily at steps and kinks, leading to expanses of flat surface. In contrast to these smooth growth mechanisms, at much higher supersaturation, ΔGs is lowered to the point where deposition is favourable even on flat surfaces (below left). This leads to adhesive or rough growth:
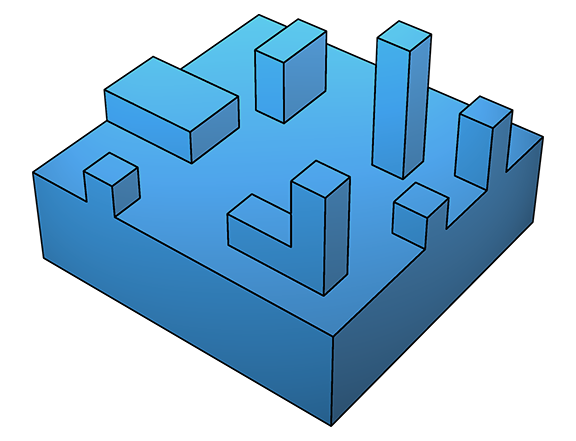
Subsequent deposition does not need a step or kink, leading to localized peaks and hillocks (above right), characteristic of a rough surface. As with spiral defect growth, two-dimensional and rough adhesive growth are also observable by atomic-force microscopy.
We have now established qualitative criteria for the physical requirements of crystals (size, shape, and quality), briefly covered the thermodynamics and kinetics of crystal growth, and described some of the major mechanisms by which crystals grow.
2: Crystal size, shape, and quality
3: Physical chemistry of crystal growth
4: Crystal growth mechanisms
Return to the main Tutorials page or to the main X-Ray Lab page