2: Crystal size, shape, and quality
X-rays are scattered by electrons, so diffracted intensities are proportional to the number of electrons encountered by the X-rays. It might seem that big crystals would always be best, but larger crystals can pose problems. They could extend beyond the X-ray beam cross-section, for example. Crystals also absorb X-rays to an extent that depends on their size and composition, so there is always a trade off. The key is to know enough to quickly select the best crystal while at the microscope. With experience it becomes quite easy for some, but it is not always obvious to begin with. Criteria for X-ray analysis include:
i ) Crystal size:
A typical sealed-tube or rotating-anode X-ray generator using a beam tunnel with half millimetre apertures gives an X-ray beam with a 'sweet spot' of fairly uniform beam intensity about a third of a millimetre in diameter. Thus, a well-centered crystal no larger than about 0.3mm would be uniformly bathed in the X-ray beam as it rotates, like this:
A modern microsource X-ray beam can be much narrower, on the order of 0.1mm or so. Thus, it is now common for crystals to be larger than the beam cross-section. As such, the irradiated volume is often less than the actual crystal volume. For a non-spherical crystal it depends on the crystal orientation:
This can lead to unpredictable data scaling problems for non-spherical crystals. In addition, accurate centering of the crystal becomes much more important. Luckily, modern data reduction and scaling programs are able to account for much of the resulting intensity variation. Nevertheless, it is always best practice to select a crystal that is as appropriate as possible for the X-ray beam at hand.
ii ) Crystal shape:
From an irradiated volume perspective, blocky crystals are better than plates, which are better than needles. The ideal shape would be spherical, as that eliminates orientation dependence. Of course, few crystals are even close to spherical! In the following schematic, the closer a crystal is to the top, the better.
 sphere
sphere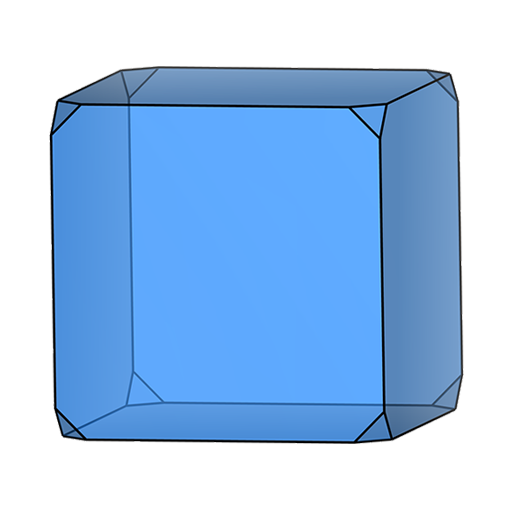 block (cube)
block (cube)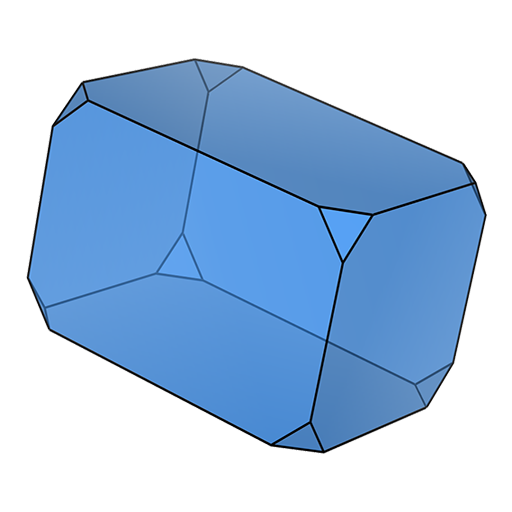 block (cuboid)
block (cuboid) slab
slab tablet
tablet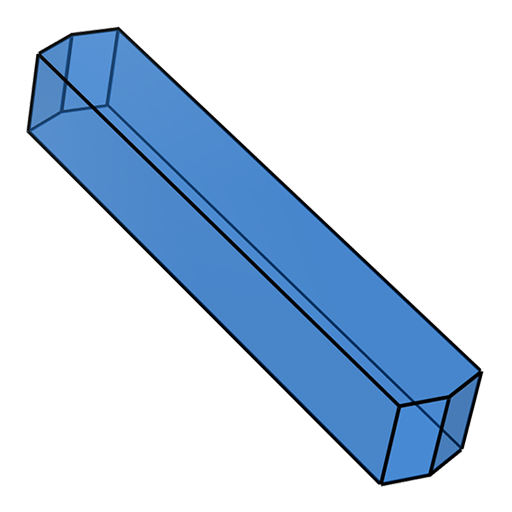 rod
rod plate
plate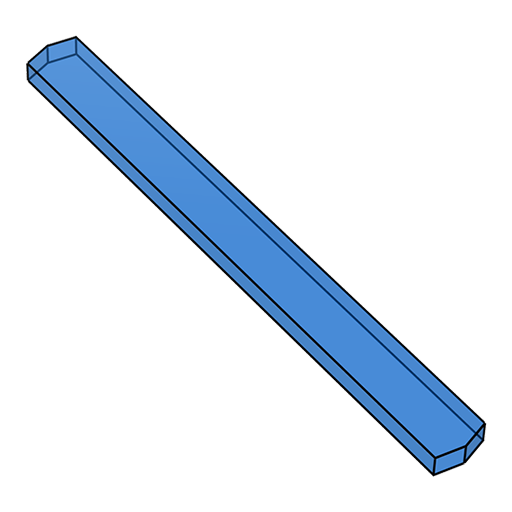 lath
lath needle
needleiii ) Crystal quality:
With improvements in hardware and software, many crystals that would have been rejected in the past can now give usable data. Still, it will probably always be the case that single crystals give the best results. Often, crystals grow as aggregated clusters, which require microsurgery to separate out single-crystal fragments. Twinned crystals can pose more of a problem.
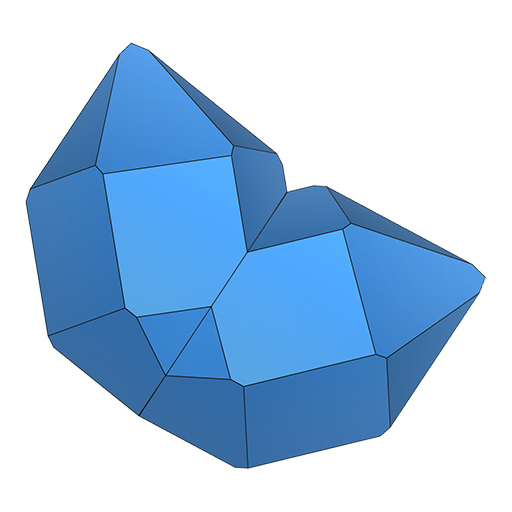 contact
contact polysynthetic
polysynthetic penetration
penetrationFor many twins it is possible to cut or break off single-crystal fragments. Nowadays, even twins that cannot be separated can still yield perfectly acceptable results.
Solution-grown crystals often incorporate solvent molecules as an integral part of the structure. If such crystals dry, they can lose solvent, which destroys long-range order, often rendering them useless. Since it is not usually possible to tell a priori whether a batch of crystals have incorporated solvent, a general rule-of-thumb is to never allow them to dry. Without good reason, they should always be kept in their mother liquor prior to selection for data collection. With that said, there are a few visual clues to indicate solvent loss. Fresh crystals that have shiny smooth surfaces tend to become hazy and develop surface striations and internal cracks on solvent loss.
Now that we have established some desirable qualities that we'd prefer crystals to exhibit, we'll turn in the next section to an overview of the physical chemistry of crystal growth.
2: Crystal size, shape, and quality
3: Physical chemistry of crystal growth
4: Crystal growth mechanisms
Return to the main Tutorials page or to the main X-Ray Lab page